Recently, National Medical Products Administration official website showed that the application for listing of metformin tablets (Ⅰ), (Ⅴ) and (Ⅴ) submitted by Hunan Huize Biomedical Technology/Hunan Jiudian Pharmaceutical Co., Ltd. was accepted by CDE. On April 11th, Zhejiang Huahai Pharmaceutical Co., Ltd. also submitted the application for listing of metformin tablets (VI) and (V) and was accepted.
China is the largest country in the world, and the number of patients ranks first in the world. The incidence of diabetes is increasing and showing a trend of younger age.
According to the data of the International Diabetes Federation (IDF), the number of adult diabetic patients in the world reached 537 million in 2021. As the country with the largest number of diabetic patients in the world, the number of diabetic patients in China increased from 90 million to 141 million in the past 10 years (2011 -2021), with an increase of 56%, equivalent to 13% of adults suffering from diabetes, and there are about 72.83 million adults.
As the pathogenesis of type 2 diabetes mellitus (T2DM) is complex, with the progress of the disease, single oral hypoglycemic drugs often cannot maintain ideal blood sugar control for a long time, and there will be many complications. More than 60% of patients need to be combined with other hypoglycemic drugs. Compound hypoglycemic preparation has the advantages of strong efficacy, high safety, convenient use, high compliance and high cost performance, and is widely used at present.
Metformin is suitable for adult patients with type 2 diabetes who are treated with eglinide and metformin hydrochloride. It is a simple compound single-tablet drug. Engelgin belongs to sodium-glucose cotransporter 2(SGLT-2) inhibitor, which can reduce the glucose reabsorption in kidney. Metformin hydrochloride can improve insulin sensitivity by increasing the intake and utilization of peripheral sugar.
The mechanism of the two hypoglycemic components is complementary, which can provide more powerful and lasting blood sugar control, and can reduce the number of tablets taken by patients, thus increasing the compliance of patients, and the improvement of compliance is also helpful to further improve blood sugar control.
The original research of metformin englejing was conducted by Boehringer Ingelheim and Lilly. In 2015, it was approved for listing by the US Food and Drug Administration, and in 2019, it was approved by National Medical Products Administration to enter the domestic market under the trade name of Ou Shuangjing.
In June, 2021, Hangzhou Zhongmei Huadong Pharmaceutical Company’s Metformin Engglinide Tablets (I) was approved to be listed in National Medical Products Administration, becoming the first imitation in China, and was exclusively included in the National Medical Insurance Catalogue Class B through medical insurance negotiation in December of the same year. The medical insurance price was 1.21 yuan/tablet (each tablet contained 500mg of Metformin Hydrochloride and 5mg of Engglinide). At present, Hangzhou Sino-American Huadong Pharmaceutical Co., Ltd. is still the only enterprise in China that has been approved to produce metformin.
According to the IQVIA database, the global sales of metformin Engel in 2020 was 505 million US dollars, an increase of over 30% compared with 2019.
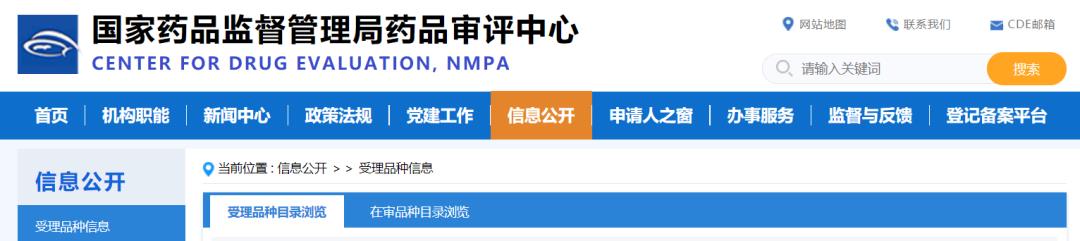
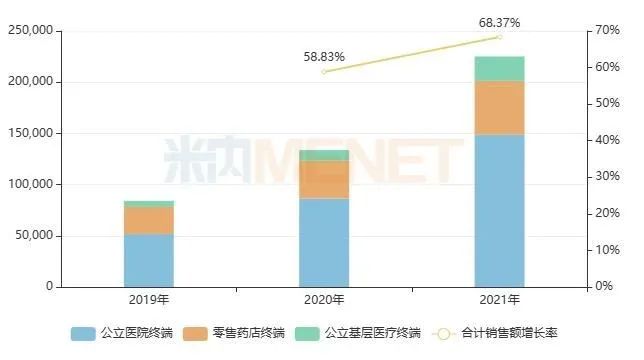
In recent years, the sales scale of oral compound hypoglycemic agents in China is also growing rapidly. According to the data of Minenet, the market of oral compound hypoglycemic agents has increased from 800 million yuan in 2019 to over 2.2 billion yuan in 2021, with the growth rate as high as 58% and 68% in 2020-2021. The market potential of oral compound hypoglycemic agents is exploding.
According to Frost&Sullivan data, the global diabetes drug market will reach 69.7 billion US dollars in 2020, and the Chinese diabetes drug market will reach 63.2 billion yuan. It is expected to reach 100 billion-level market in 2024, and will grow at a high speed with a CAGR of 10.4% from 2020 to 2030. The diabetes field has become a field that major pharmaceutical companies will strive for.
Hypoglycemic drugs are constantly innovating, and they don’t stop rolling up. Take Engelje as an example. At present, there are 5 domestic enterprises with production approval, and 18 enterprises are in the production reporting stage. It should be noted that Engelje tablets have been included in the fourth batch of national adoption in February, 2021, which shows the expectations of enterprises for Engelje tablets. However, judging from the global sales of Engelje (US$ 8.2 billion in 2022), the domestic market is far from saturated (over 400 million yuan in 2021).
Under the condition that the single drug track is crowded, some enterprises began to lay out compound preparations. Since this year, seven enterprises have submitted the application for the listing of metformin engeglinide tablets/sustained-release tablets, plus two in 2021, at present, a total of nine enterprises are in the production reporting stage of metformin engeglinide.
It is worth mentioning that although the sales of metformin monotherapy have declined in recent years, it can still be seen everywhere in the market, especially in pharmacies.
At present, the best-selling hypoglycemic compound preparations in the market are mainly metformin plus another kind of hypoglycemic drugs, such as "metformin+sulfonamide urea derivatives", "metformin +DPP-4 inhibitor" and "metformin +SGLT2 inhibitor". In addition, there are more and more combinations of compound hypoglycemic preparations, such as "SGLT2 inhibitor +DPP-4 inhibitor", which has been reported for production by enterprises.
END Author | Ryan Source | Medicine Chunqiu