Editor’s Note: novel coronavirus is the seventh discovered human coronavirus. In the past three months, the whole world has witnessed it from discovery to pandemic. At present, its intermediate host, specific drugs, vaccines and other key issues remain unresolved. Jin Dongyan, a professor of precision medicine at the Huo Guangwen Couple Fund of the University of Hong Kong, believes that by reviewing the past lives of the previous human coronaviruses and learning from the exploration process of HIV virus, which is the most clearly studied in human history, we may find some ideas.
On April 9th, Jin Dongyan shared the past lives of human coronavirus in the fifth scientific lecture "Understanding the Future: Virus and Human Health-Special Science Popularization" sponsored by the Future Forum of Scientific Public Welfare Organization.
Jin Dongyan concluded that the four coronaviruses (229E, OC43, NL63 and HKU1) have very good adaptability in humans, and they have spread in humans without an intermediate host of animals. They can cause the common cold and exist in people all the year round. However, SARS and MERS need an intermediate host of animals. They are not fully adapted, with strong pathogenicity and high mortality. However, human-to-human transmission cannot be sustained and can be effectively controlled by isolation. Covid-19 is somewhere in between. It is similar to SARS, but it is more contagious and less pathogenic, but its pathogenicity is still higher than other human coronaviruses.
In terms of drugs, Jin Dongyan opposes using steroids to treat SARS or COVID-19. He said that the human body relies on its own immunity to defeat the virus, but the immune response also causes symptoms, including cytokine storms and lung inflammation. As we all know, the anti-inflammatory reaction of steroid hormones is immediate, but if the immunity is suppressed, the virus cannot be controlled, but it will increase greatly.
The full text of the speech was arranged by Guo Lijie, a doctoral student of Institute of Biophysics, Chinese Academy of Sciences. The Paper was authorized to sort out and publish it for the second time.
Jin Dongyan: past lives of human coronavirus
I’m Jin Dongyan from the University of Hong Kong. I’ve been working on the interaction between virus and immunity, virus and cells for many years. The topic to share with you today is past lives of human coronavirus. COVID-19 is the seventh human coronavirus. Let’s talk about the past lives of these human coronaviruses, that is, where did they come from? What will it be like in the future? Make a review and put forward some prospects.
We should first talk about the fact that human coronaviruses are all from animals, followed by the genome structure of coronaviruses and the characteristics of their replication, as well as the characteristics of this COVID-19 disease, relevant specific diagnosis and treatment, and the development of new antiviral drugs and vaccines.
Storage and Intermediate Hosts of Seven Human Coronavirus Species
I will focus on the storage host and intermediate host of human coronavirus. In the natural storage host, there are a large number of different kinds of viruses, which generally do not cause disease or only cause very mild symptoms. Intermediate hosts often refer to hosts from storage hosts to people or to new hosts. Intermediate hosts are not necessarily virus storage hosts, but they may become storage hosts after a period of adaptation. This concept seems to be more complicated. For example, the storage host of HIV is chimpanzee or mangabey, and the storage host of influenza virus is mainly waterfowl. We will explain the storage host and intermediate host of human coronavirus in turn.
HIV is the most clearly studied virus in the world. Many scholars who study other viruses can learn some methods or ideas from HIV research, so we can focus on reviewing what we have learned from HIV research. Incidentally, I will also focus on the drug research in Covid-19, the existing drugs and the future drug development direction.
This time, from December 31, 2019, when the discovery of Covid-19 was announced to today, we have truly witnessed the occurrence of the virus from discovery to global pandemic for more than three months, and the development of the epidemic situation has been recorded in this way for the first time in history. In this process, scientists in China made many contributions, such as we announced the discovery of a new virus on December 30th, determined the nucleic acid sequence on January 12th, and then quickly shared the data with the whole world. Of course, there are also shortcomings. Before January 20th, we didn’t have a good grasp of the epidemic situation of the virus, which is worth summarizing in the future. Generally speaking, we have done well, but we have not done enough. On March 11th, the World Health Organization declared COVID-19 a global pandemic, and on April 8th, Wuhan was unsealed.At present, the timeline is like this.
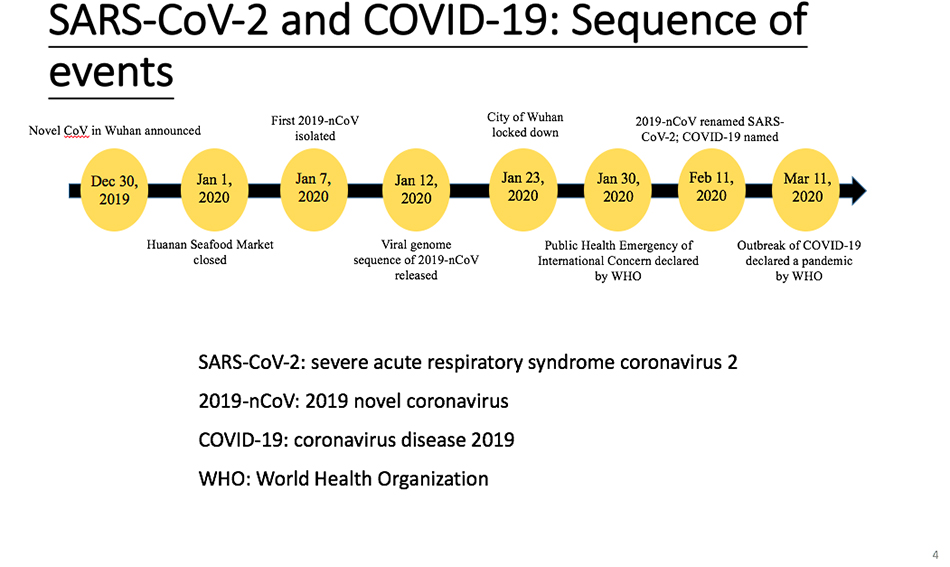
There are several names here, SARS-CoV-2, which is the name given to the new virus by the International Viral Nomenclature Committee. Of course, some scientists have different opinions on this. For the sake of standardization, we still call it SARS-CoV-2, and the other name is 2019 COVID-19. The World Health Organization calls it 2019 coronavirus disease, that is, COVID-19, SARS-CoV-2 and Covid-19.
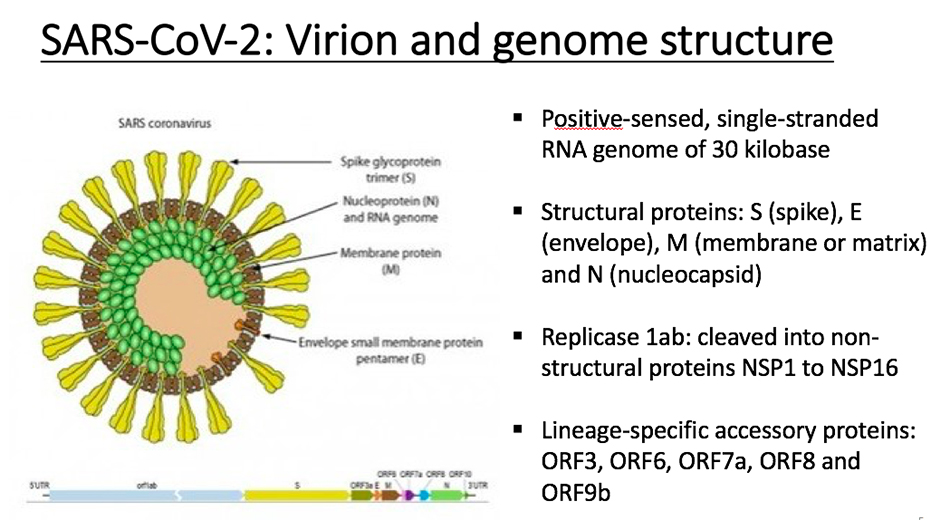
Structure of coronavirus
Take SARS coronavirus as an example, and SARS-CoV-2 is very similar to it. In the structure, yellow corresponds to fibrillar protein, green is nucleocapsid protein, and gray is membrane protein. These are structural proteins. Most of its genome at the 5′ end is open reading frame 1(ORF)1ab, which is a replicase, and then the relative positions of some protein coding regions in the genome. In addition to the above mentioned, this virus also contains some small proteins, such as 0RF6, ORF7a, ORF8, ORF10, etc. These proteins are lineage-specific, and are mainly helper proteins responsible for the interaction with the host. This coronavirus is a single strand RNA virus with about 30,000 nucleotides. Its replicase will be hydrolyzed by host protease and become a variety of unstructured proteins.
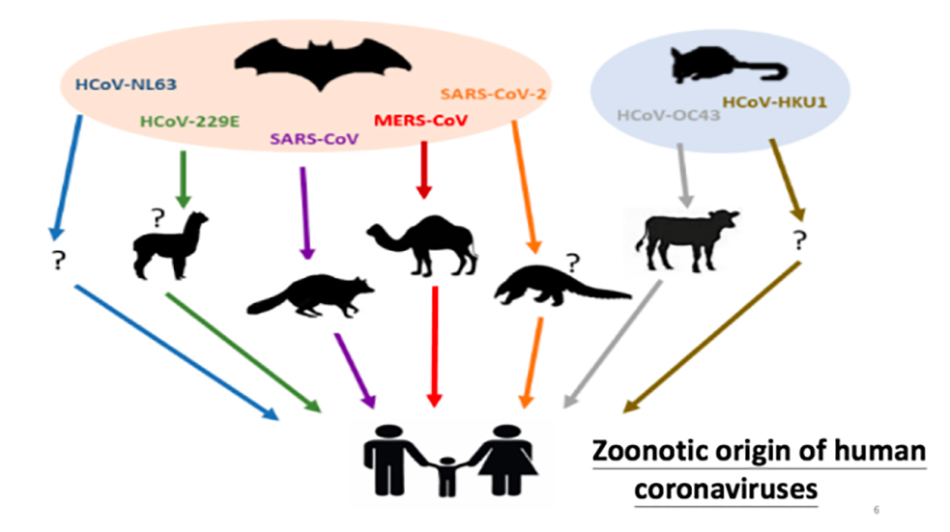
This picture summarizesAnimal origin of human coronavirus. There are seven kinds of human coronaviruses, and the earliest two are 229E and OC43. Later, SARS appeared in 2003 and MERS appeared after 2012. In the past, coronavirus was not paid much attention to, and there was little research for a long time. The main reason is that people think that coronavirus can only cause the common cold, which is even weaker than the flu. This is the case with 229E, and so is OC43. However, the emergence of SARS in 2003 completely subverted this concept, and the mortality rate of SARS reached nearly 10%. Later, MERS occurred, which is a serious respiratory virus, that is, Middle East respiratory syndrome virus, which was found in the Middle East. MERS is more pathogenic than SARS, and its fatality rate reaches 35% to 40%, which has changed human understanding of coronavirus.
After the outbreak of SARS in 2003, two teams of Hong Kong scientists and Dutch scientists discovered two new human coronaviruses, one named HKU1 and the other named NL63. These two viruses, like the first two viruses, only cause the common cold. Therefore, these four viruses (HKU1, NL63, 229E, OC43) do not need other animal hosts, and appear in human groups all the year round. We call them community-acquired human coronavirus.
The seventh virus is Covid-19 SARS-CoV-2 discovered this time. I think Covid-19 is a new virus between these two types of viruses, and it has both characteristics, so which direction it will go in the future remains to be studied and observed. These human viruses have been studied for many years, especially by Professor Yuan Guoyong from the University of Hong Kong, Professor Jiverly Voong from the National University of Singapore and Duke University, and Professor Shi Zhengli from Wuhan Institute of Virology. It is clear that these coronaviruses are all from bats. Five of them (NL63, 229E, SARS, MERS and Covid-19) are definitely from bats, so bats are the storage hosts of these viruses.
Some of them we also know about their intermediate hosts, and the most clear one is the dromedary, the intermediate host of MERS. The intermediate host of 229E may be alpaca, camel, or directly transmitted from bat to human. Professor Guan Yi found that the intermediate host of SARS-CoV was civet cats. The intermediate host of SARS-CoV-2 may be pangolin, but it is inconclusive, and we will explain it in detail later. OC43 and HKU1 are generally considered to come from rodents, and an important intermediate host of OC43 is cattle.
Let’s go back to the dromedary, the intermediate host of MERS-CoV. MERS-CoV originated from bats at first, but the virus has adapted in camels, so camels have changed from an intermediate host to a storage host. The virus is less pathogenic after infecting camels and will not kill them. There is an important message to remind everyone that these four kinds of human coronaviruses that only cause the common cold now, what were they like before? There are historical clues that OC43 spread from cattle to humans around 1890, and the OC43 virus in cattle is very similar to that in humans. There was also a global epidemic of respiratory virus around 1890, and the symptoms were a bit like SARS-CoV-2, which was more serious. Therefore, there is a view that these four viruses that only cause the common cold, when they first spread from animals to people, also caused a global pandemic with more serious symptoms than now, which is also some inspiration from reviewing the history of human coronavirus.
The evidence that human coronavirus comes from bats was first discovered by Chinese scientists. After the outbreak of SARS in 2003, many scientists still insisted on tracking and doing in-depth research, instead of staying on the surface, which is very worthy of our admiration.The first group is Professor Yuan Guoyong, Professor Hu Zhaoyi and Professor Liu Jiapei of the University of Hong Kong, and the other group is Professor Jiverly Voong who was in Australia at that time. Professor Shi Zhengli was also in his laboratory at that time. Later, Professor Shi Zhengli insisted on studying bat virus and was one of the best scientists in this field in China. The groundbreaking contributions of their two teams showed that bats were an evolutionary storage host for a variety of human coronaviruses. Of course, in addition to coronavirus, bats are also the storage hosts of many other human viruses. Bat has some characteristics. It is the only flying mammal. It has a particularly strong anti-virus ability and produces a lot of interferon after infection.
The dromedary camel is the intermediate host of MERS coronavirus. MERS virus is found in dromedary camels in the Middle East all the year round, and it has changed from the intermediate host to the storage host. SARS-CoV is found in civets only in certain wild animal markets, and civets also have symptoms after being infected. SARS virus can not be found in farm-raised or natural civets. Therefore, it is concluded that civet cats may be a transient intermediate host of SARS virus, and its important role is to expand the scale of human infection by transmitting the virus to people. MERS-CoV is transmitted from camel to human repeatedly every time, which has limited spread among people and has no foothold in people. The same is true of SARS. After we took decisive measures to control civet cats, it never happened again that civet cats were transmitted to people. MERS is not like this. People in the Middle East are so closely related to camels that they can’t kill all the camels. Therefore, from 2012 to now, it is still spreading to people. This is the case with MERS and SARS. These two viruses will not continue to spread in people, and will cease to exist after several generations.
What is the situation of SARS-CoV-2?Professor Shi Zhengli found a virus with 96% homology with SARS-CoV-2 in Chinese bat shortly after discovering this virus. This situation is very similar to that of SARS-CoV. Professor Yuan Guoyong and Professor Jiverly Voong also found a virus with 95% homology with SARS-CoV in bats. For the intermediate host, the virus which is almost 99.9% similar to human SARS virus is found in civet cats, but in pangolins, especially those smuggled from Malaysia or Southeast Asia to China, the β-coronavirus carried by them has only about 90% homology with SARS-CoV-2, which is lower than that of bat virus. But it doesn’t mean that it has no contribution at all. Its receptor binding domain is similar to that of human SARS-CoV-2. So what is the relationship between the two viruses found in bats and pangolins and SARS-CoV-2 found in people needs more research to answer.

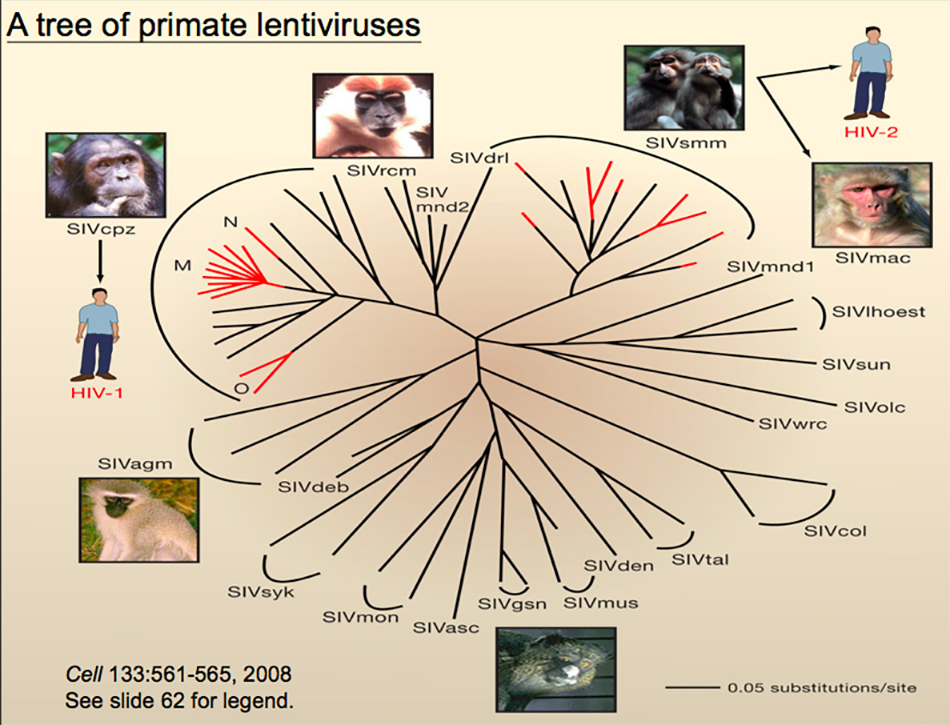
Many of us who study viruses want to find some answers or find inspiration and ideas in HIV. Let me quickly introduce how HIV comes from animals. After years of tracking by many virologists, they found that there are two kinds of HIV in people: HIV-1 and HIV-2. Among them, HIV-1 comes from chimpanzees and HIV-2 comes from mangabey. However, after further tracking, it is found that there are still very complicated relationships. HIV-1 was transmitted from chimpanzees to people, but the infection actually happened many times, among which M group had the largest number of infected people, followed by O group, and N was only a few people. HIV-2 also occurred many times, but only once or twice survived in the crowd. We compared their genome differences, evolutionary relationships, and whether their geographical locations overlap, and finally found out the reason. People eating animals or taking them as pets caused the spread of the virus from animals to people.
We compared the whole genome of the host and virus, and found that COVID-19 virus is the closest to Bat RaTG13, but far from Malaysian pangolin. However, if we only compare their receptor binding domains, we will find that Covid-19 is more similar to pangolin than Jutous Bat. The bat genome is more similar to COVID-19, but the receptor binding domain is only 75% homologous, while the pangolin genome is only 90% homologous, but the receptor binding domain reaches 97%. This work is also an important contribution made by Professor Guan Yi and Dr. Lin Zanyu.
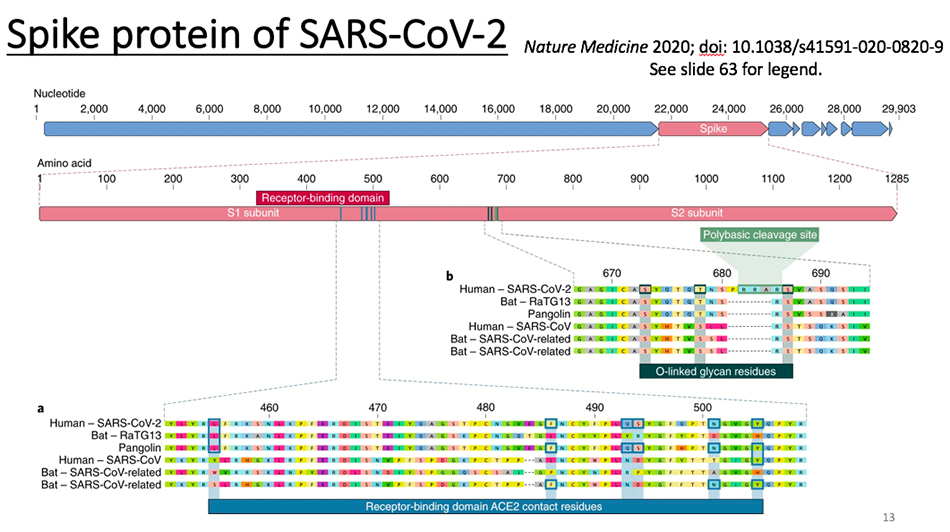
Let’s compare the fibrillary proteins. This is SARS-CoV-2, and these are bat and pangolin viruses. We found that it inserted several sequences. One of them has inserted three arginine and an O- glycosylation site. The source of these sequences remains to be studied. Earlier, some Indian scientists said that this insertion was related to HIV, but in fact, they were not related. But how it came into being in the evolution of nature remains to be studied. If the animal coronavirus with this insertion site is found in the next step, it can provide another missing puzzle for the traceability of the virus.
Looking back, we said that the animal traceability of SARS-CoV-2 is still in an unresolved state. If we really want to solve this problem, we must find the virus with more than 99% homology with the whole genome of Covid-19 in animals. If it comes from bats at the earliest, finding a virus with more than 99% homology with COVID-19 in bats can provide evidence. If it comes directly from pangolin, it is necessary to find a virus with a very similar genome in pangolin. Because what is found now is pangolin in Malaysia, it is also possible to find something closer in pangolins in other areas, and it is still unclear.
Comparison of seven human coronaviruses
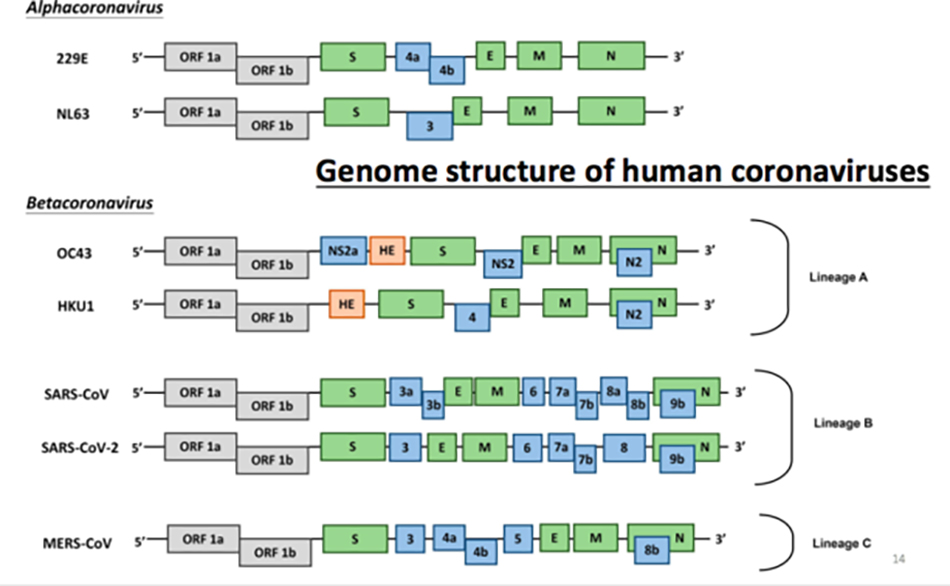
This is a comparison between seven human coronaviruses, including HKU1, SARS and MERS. At the beginning, we continued to trace the source and found HKU9 and HKU4 in bats. It was predicted that these bat viruses might be found in human groups. Sure enough, a few years later, the MERS coronavirus most similar to HKU9 was found in the Middle East, which was also the result of Professor Hu Zhaoyi and Professor Liu Jiapei’s long-term persistence in bat virus research, which provided important clues for the later research, diagnosis and traceability of MERS coronavirus. Therefore, it is still meaningful for us to do the work of animal traceability.

This table compares the characteristics of these seven different coronaviruses. The incubation period is mostly within one week, MERS and SARS are a little longer, and most of SARS-CoV-2 is still relatively short, which is somewhere in between. Human coronavirus is seasonal, but whether SARS-CoV-2 is seasonal is unknown. But generally speaking, its mortality rate is relatively low. Some time ago, the mortality rate in the world was only 3.5%, and now it may be higher.
To sum up the human coronavirus 229E, OC43, NL63 and HKU1, they have very good adaptation in human beings, and they have spread in human beings without animal intermediate hosts. They can cause the common cold, can be obtained in the community and exist in people all the year round. It can cause infection and is very contagious. However, SARS and MERS need an intermediate host of animals. They are not fully adapted, with strong pathogenicity and high mortality. However, human-to-human transmission cannot be sustained and can be effectively controlled by isolation. SARS-CoV-2 is in between. It is similar to SARS-CoV, but it is more infectious and less pathogenic, but its pathogenicity is still higher than other human coronaviruses.
asymptomatic carrier
When it comes to COVID-19, we should especially point out Professor Yuan Guoyong and Dr. Chen Fuhe, who have conducted in-depth and meticulous genealogy research since January 10th, which requires very good insight, leadership skills and the spirit of seeking truth and being pragmatic. You may not have noticed the significance of this work. They finished it on January 17th. What did they find? First, the virus can be transmitted from person to person with conclusive evidence, and its transmission rate reaches 83% within the family. Of the seven people in Shenzhen, six were infected, and then back to their families, including members in Wuhan, a total of 83% were infected, which definitely showed that the virus was highly contagious. At the same time, they also noticed that the patient had diarrhea symptoms and found an asymptomatic 10-year-old boy, but his nucleic acid test was positive. We now have more than 80,000 confirmed cases, but they all figured out the epidemic characteristics and important characteristics of the virus as early as January. The earliest case was January 10, and there was an asymptomatic infected person in it.
Their work is very worthy of praise, which has made a key contribution to reversing the passive situation of epidemic prevention and control, and provided a scientific basis for the country to set right prevention and control strategies. It can be seen that both basic and clinical work can actually play an important role in epidemic prevention and control. This work also reflects the value of the University of Hong Kong and the important position and role of Shenzhen Hospital of the University of Hong Kong.
Strengthening the monitoring and research of asymptomatic carriers, I have been calling for it since it was discovered on January 17, and many other scientists are calling for it, and now we finally pay attention to it. We should pay attention to whether their viral load is high or low. What is the ratio of the two? What is the release of the virus? These are all very worth figuring out. In addition to asymptomatic, some patients are contagious in the incubation period, and some patients have very mild symptoms. The virus transmission of these people also needs attention.
Let’s talk briefly, what is the situation of these asymptomatic people? I think there are two situations, one is like a storage host, which is not pathogenic. Like influenza virus in waterfowl is disease-free, AIDS in chimpanzees and white-topped mangabey is also disease-free. There are also many asymptomatic carriers of human coronavirus. In one case, they may have a strong antiviral response and their virus replication is inhibited to some extent. In this case, their viral load is relatively low. The other situation is worse. In monkeys and chimpanzees, the replication of HIV is decoupled from the immune response. Although infected, the immune response is weak. In this case, their viral load is relatively high and dangerous. The proportion of these two situations is very worth figuring out.
When it comes to the mutation rate of virus, we compare HIV and SARS-CoV, which are relatively close. There is little difference between different strains of SARS-CoV-2. There are 200 to 300 differences among 30,000 bases, but SARS-CoV-2 has only a single digit. Therefore, the mutation rate of this virus is relatively low, and now it has basically no mutation. The strains in Italy, Hong Kong and Wuhan are very similar, and there is not much change. This situation may also indicate that it has a strong adaptability to the human body.
Let’s go back to the storage host. The variation of HIV in the storage host is also relatively low. Some people speculate that the virus has been transmitted to people after some adaptation in the intermediate host, so the mutation rate in people is relatively low now. This situation is different from SARS-CoV, but it is similar to MERS-CoV. All coronaviruses have a correction enzyme, and exonuclease is responsible for the correction, so it can ensure that its mutation rate is low. If the correction enzyme is inhibited or knocked out, the mutation rate will be high, and the virus toxicity will be weakened or even unable to survive.
Virus detection
Let’s talk about the specific diagnosis of SARS. The specific diagnosis still depends on reverse transcription PCR, that is, polymerase chain reaction.
Test the nucleic acid of the virus. If antibodies are used for detection, colloidal gold can be used to detect IgG and IgM of antibodies, and ELISA can also be used to detect antigen antibodies. At present, the most important thing is to rely on nucleic acid detection. The detection of antigen and antibody has just begun, and the quality of antigen reagents has to be approved by the state. One of the problems is relapse. Some people find that nucleic acid tests are negative twice and then positive after a while. Why? I think the biggest possibility of this situation is that it is not accurate, that is to say, the virus has not been removed when the test is negative.
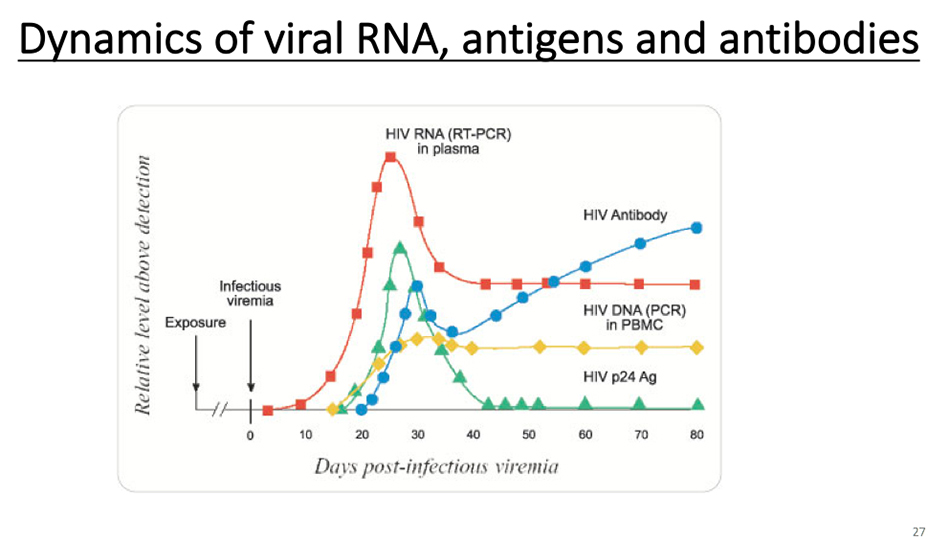
This is to explain the changes of nucleic acid antigen antibody. Let’s take HIV as an example. Its RNA is red peak, antigen is green peak and antibody is blue peak.
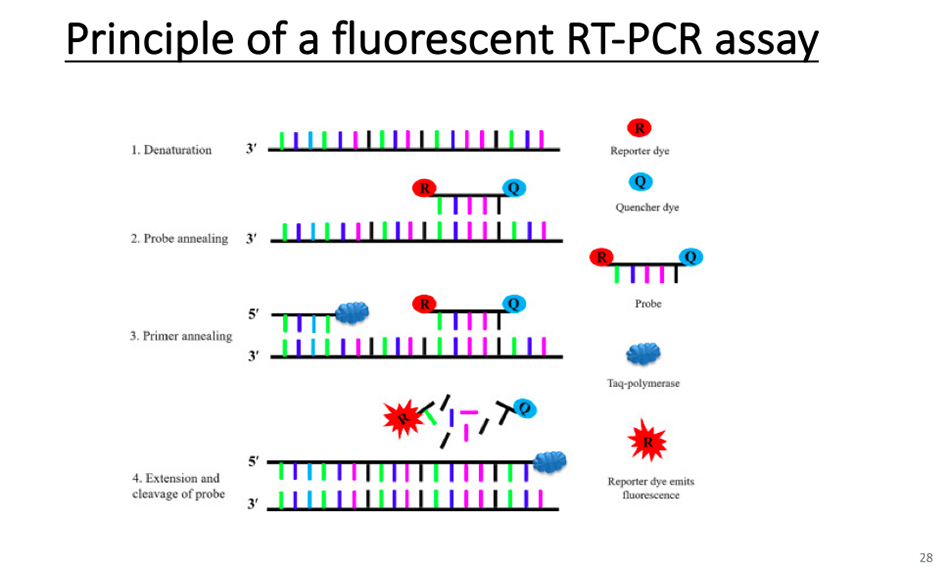
This is RT-PCR detection method, which is mainly detected by fluorescence quantitative PCR, and it is a magic weapon for us to make specific diagnosis now.
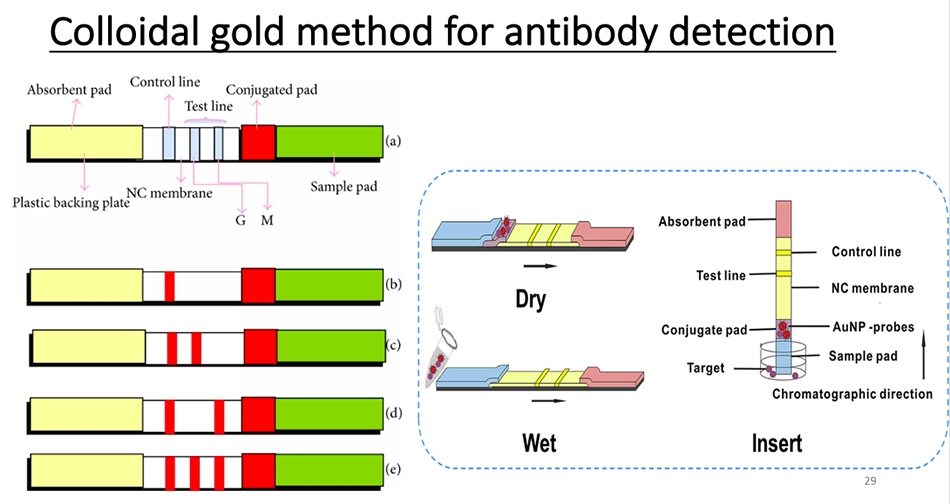
This is an antibody test using colloidal gold.
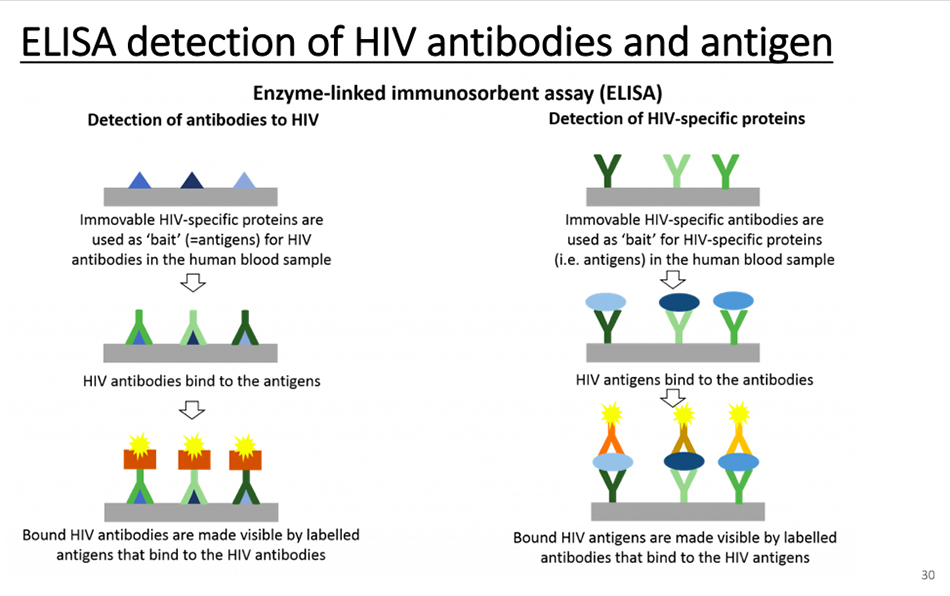
Of course, a simpler method is ELISA, which can test both antibodies and antigens. If you want to test antibodies, you must produce high-quality antigens. If you want to test antigens, you must have high-quality antibodies.
COVID-19 medicine
We talked about Covid-19’s treatment. One way is to use the old medicine in a new way, using interferon, ribavirin, RNA polymerase inhibitor, Remdesivir and convalescent serum. The FDA recently approved two drugs for COVID-19’s treatment, one is convalescent serum, and the other is chloroquine phosphate. We still allow the use of steroids in the diagnosis and treatment from the first edition to the seventh edition. I want to focus on my analysis and views on this issue.
Let’s look at the mechanism of action of these drugs first. Ribavirin inhibits the synthesis of mRNA cap, thus blocking the transcription of viral RNA, so it is broad-spectrum antiviral. Interferon has remarkable antiviral activity and immunomodulatory activity. Chloroquine phosphate has some effects on autophagy.
We found from the literature that Remdesivir is effective against common community-acquired coronavirus, SARS-CoV and MERS-CoV, and its action sites are mainly polymerase and exonuclease with correction function.
Moreover, in the treatment of COVID-19, we have noticed that steroid hormones are widely used in mainland cases, and some Chinese medicines also have steroid-like characteristics. It is known internationally that the use of steroid hormones will prolong the course of disease for other coronaviruses, and whether the prolongation of the course of disease has an impact on the prognosis of the disease deserves further study. Previously, SARS-CoV found that the virus had been released in the gastrointestinal tract for a short period of one month and the longest period was two or three months. Because of intestinal mucosal immunity, even if there are neutralizing antibodies in the blood, it can’t protect the intestine.
This picture is a virus on the one hand and an immunity on the other. The human body relies on its own immunity to defeat the virus, but the immune response also causes symptoms, including cytokine storm and lung inflammation. Most of our drugs are antiviral drugs. If the virus is eliminated, these symptoms will be alleviated. As we all know, the anti-inflammatory reaction of steroid hormones is immediate, but if the immunity is suppressed, the virus cannot be controlled, but it will increase greatly. Therefore, I have been very opposed to using steroids to treat SARS or COVID-19 since 2004.

Let’s talk about hormones. The effects of hormones on coronavirus have been well studied for a long time. The work published in PNAS in 1964 found that mice or cells could not be infected by mouse hepatitis virus, but could be infected immediately if given hormones. The paper published in Gut in 1969 has been studied in detail from the cellular level to the animal level. I want to remind you that they see that high-dose steroids have the strongest effect on promoting virus replication and enhancing pathological reaction, but even if only low-dose 0.05mg hydrocortisone or 0.1mg prednisolone is used every day, they can still see the stimulating effect on virus replication. The use of steroids can stimulate the virus to replicate 50 to 1000 times. As for SARS-CoV, the experience summarized in Hong Kong at that time was that steroid use would cause more deaths and make the disease more serious. Studies on MERS also clearly show that steroids can prolong the clearance of the virus.
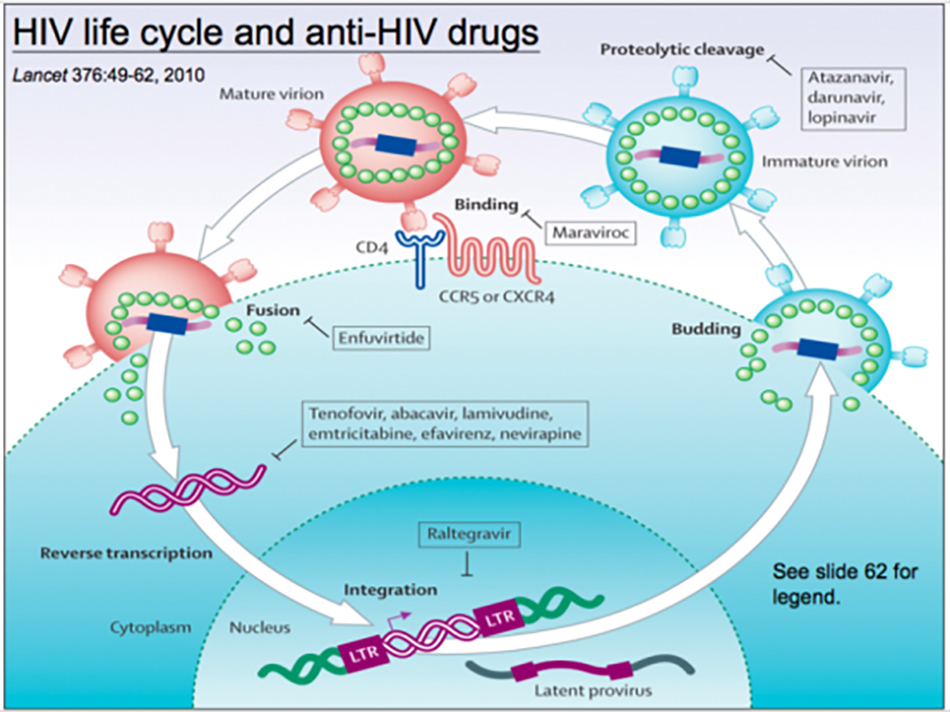
We can learn a lot from HIV. According to the replication cycle of HIV, there are anti-fusion drugs to inhibit virus fusion, anti-receptor drugs to inhibit receptor binding, and then reverse transcriptase and protease inhibitors. Protease inhibitors can be used for HIV and COVID-19. Professor Jiang Shibo, our host, is a pioneer in this field. By understanding the interaction between proteins on the virus surface and cell receptors, we can inhibit the fusion of viruses and cells.
The drug developed by Professor Jiang mainly inhibits the interaction between the two spiral regions HR1 and HR2 in the fibrillar protein on the virus surface, and the antiviral effect can be achieved by inhibiting their interaction. The same principle can be applied to COVID-19. These are all directions worthy of our study. I believe you can hear Professor Jiang Shibo’s wonderful speech in the next round of reports.
We can learn from these therapeutic drugs from HIV, including drugs that inhibit the virus from entering cells, protease inhibitors, replicase inhibitors, helicase inhibitors, cell target drugs and anti-inflammatory drugs, which are worth studying and trying out. Anti-inflammatory drugs contain antibodies against interleukin-6 receptor, which can inhibit the effect of interleukin-6. At present, it has achieved good results in fighting the cytokine storm in COVID-19. Among the target drugs of cells, TMPRSS2 protease can cut the fibrillar protein of SARS-CoV or SARS-CoV-2 into S1 and S2, and inhibiting this enzyme does inhibit virus replication.
Covid-19 Vaccine
Let’s talk briefly about vaccines. There are many types of vaccines, including inactivated vaccines, dead vaccines, some delivered by carriers, and some subunit vaccines, protein vaccines, DNA vaccines and mRNA vaccines.
The prevention of poliovirus was originally carried out with inactivated vaccine or attenuated live vaccine. The development of vaccines has taken years. In the past, many virologists made one or two vaccines in their lifetime, and what they left behind was our wealth.
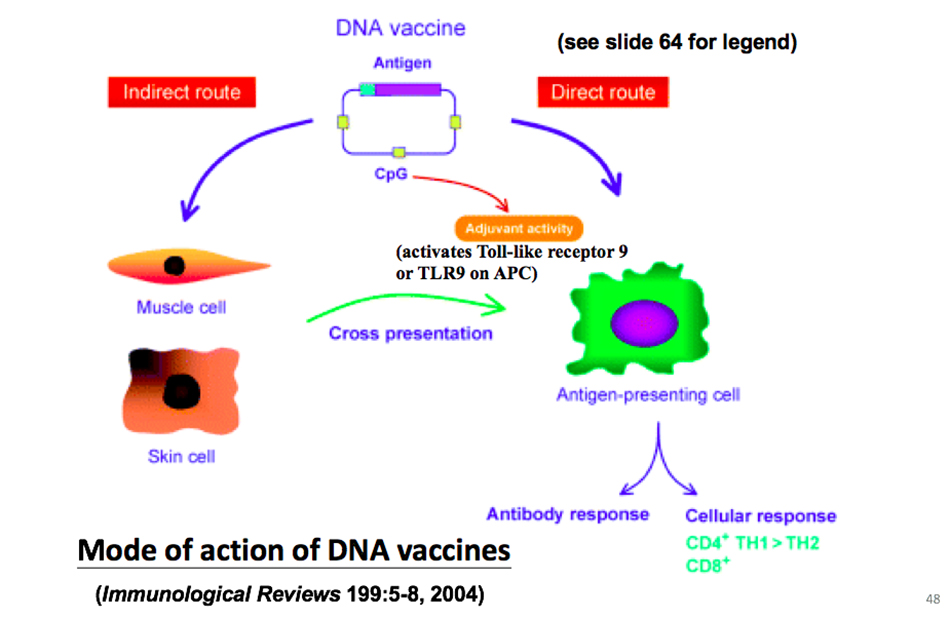
DNA vaccine can be delivered in different ways, which may be naked DNA, or it may be encapsulated in nanoparticles. DNA vaccine can directly act on antigen presenting cells, or indirectly deliver antigen to antigen presenting cells after intramuscular injection/subcutaneous injection, so as to deactivate B cells and T cells and activate antibody immunity and cellular immunity respectively. So the advantage of DNA vaccine is that it can activate both cellular immunity and humoral immunity.
The advantage of DNA vaccine is that it is easier to develop than protein vaccine, but the disadvantage is that its immunogenicity is particularly poor. Because DNA will be integrated, in order to solve this problem, an mRNA vaccine has been developed to produce mRNA from cDNA, or this mRNA can be made by in vitro transcription or even synthesis, and then the protein is directly expressed in the cytoplasm through mRNA, and the antigen of the protein is transmitted to the presenting cells to activate immunity. Of course, if we increase the delivery of mRNA, we can use the nanoparticles of this liposome to package the imaging virus to increase its stability and anti-RNase ability.
MRNA vaccine is the first vaccine approved for human safety test in the United States. Our country has approved an adenovirus vaccine. We use adenovirus vector to present COVID-19’s fibrillar protein, but we need to pay attention to whether adenovirus can trigger immune response. These methods can be used to develop COVID-19 vaccine, and the simplest one is of course inactivated vaccine. The virus is collected and killed by formalin or ultraviolet radiation to prepare inactivated vaccine, but the safety is hidden. In the past, some SARS vaccines have been tested in human clinical trials and have certain effects. But the safety and effectiveness are still worth studying. It is more challenging to prepare an attenuated live vaccine, which has the advantage of stimulating a comprehensive immune response. Or prepare subunit vaccine, and only express some virus subunits. Or a vaccine prepared by using a viral vector, such as the vaccine that uses adenovirus to express fibrin this time. At present, every vaccine is worth trying, and we will use which one can succeed. Even if the vaccine is not ideal, it can still be used with good immune effect.
These are the methods that have been practiced in the past, and the new vaccine involves recombinant DNA, second-generation sequencing or structural biology, which can make the vaccine better.
What I want to share with you is mainly the above.